Hacohen Lab
The Hacohen lab consists of immunologists, geneticists, biochemists, technologists and computational biologists working together to understand basic immune processes and immune-mediated diseases.

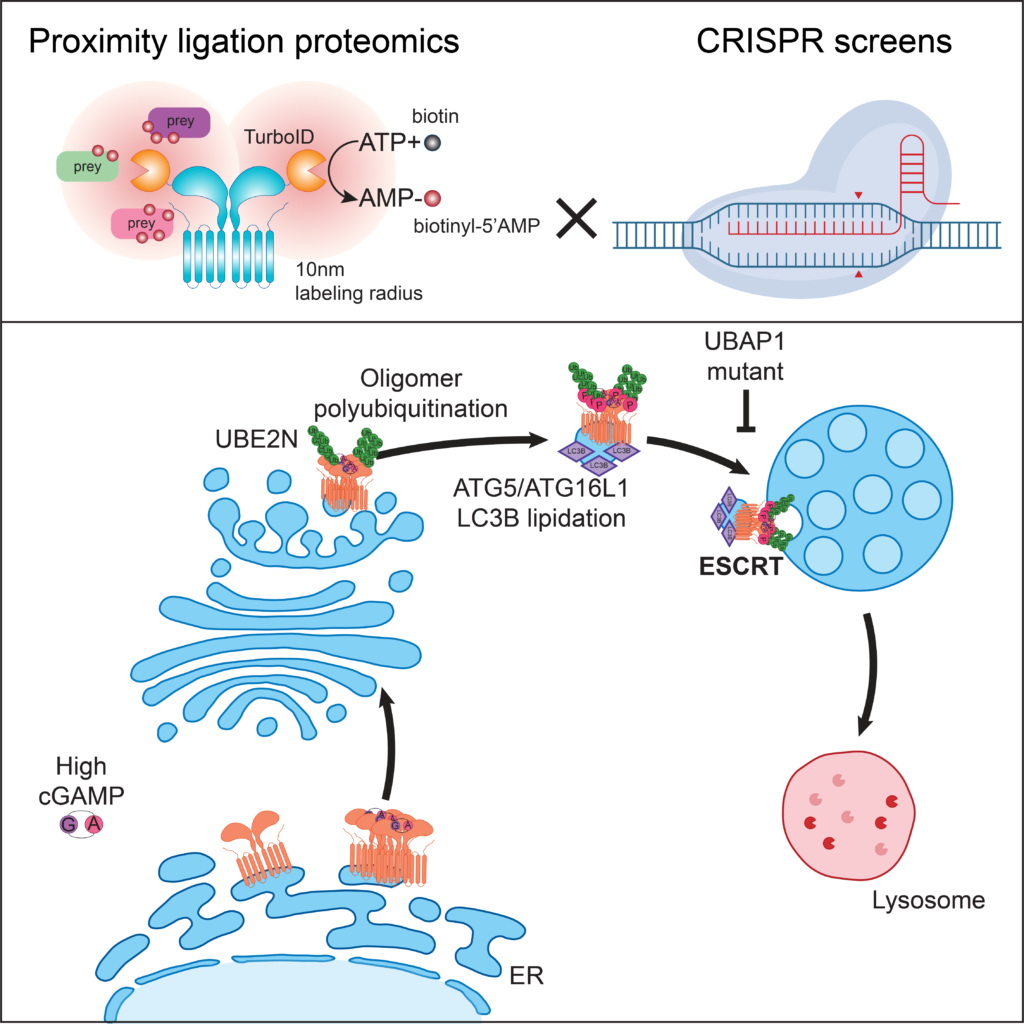
ESCRT-dependent STING degradation inhibits steady-state and cGAMP-induced signalling
Systems approaches to elucidate genes regulating STING trafficking. By combining proximity ligation proteomics and CRISPR screens, Matteo Gentili, a pdoc in the lab, showed that the ESCRT complex is required for STING degradation at the lysosome. ESCRT regulates a homeostatic STING degradative flux and an ESCRT mutant identified in patients with hereditary spastic paraplegia increases steady-state STING-dependent type I IFN responses.
pH measurement in cells
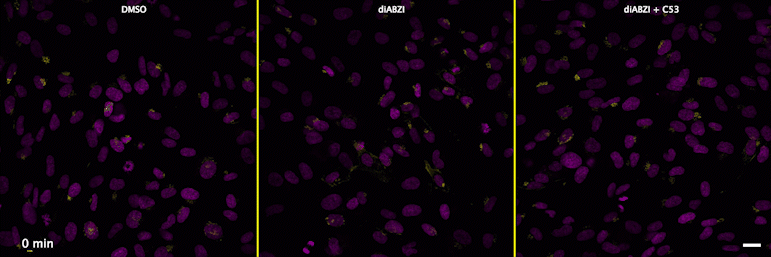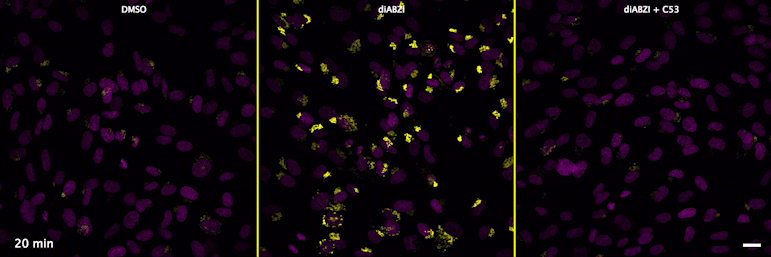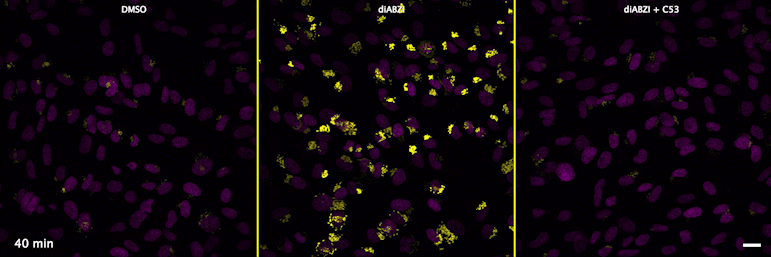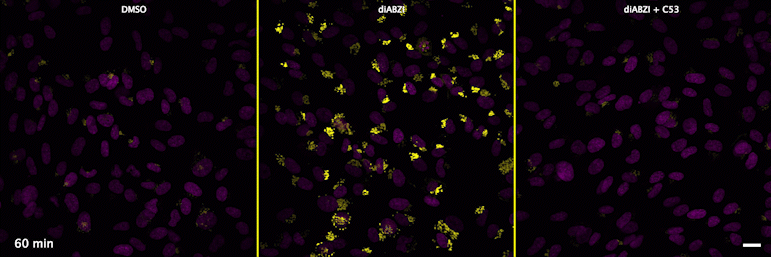
| unstimulated | STING agonist | STING agonist with channel blocker |
Human STING is a proton channel (link to Science here).
Through a convergence of several concepts, Bingxu Liu, a graduate student in our lab and Darrell Irvine’s lab, hypothesized that human STING is a proton channel that could potentially activate key cellular processes. To test this hypothesis, Bingxu, Becca Carlson and Ivan Pires carried out experiments in cells and in vitro and found that: (1) STING activation induced a pH increase in the Golgi; (2) STING reconstituted in liposomes enabled transmembrane proton transport; (3) the small molecule C53 (a STING agonist that binds the putative channel interface based on published structures) blocked STING-induced proton flux in the Golgi and in liposomes, and inhibited LC3B lipidation and inflammasome activation in cells. Sting’s role as channel is thus to trigger LC3B lipidation and inflammasome activation, processes which appear decoupled from STING’s interferon-inducing function.
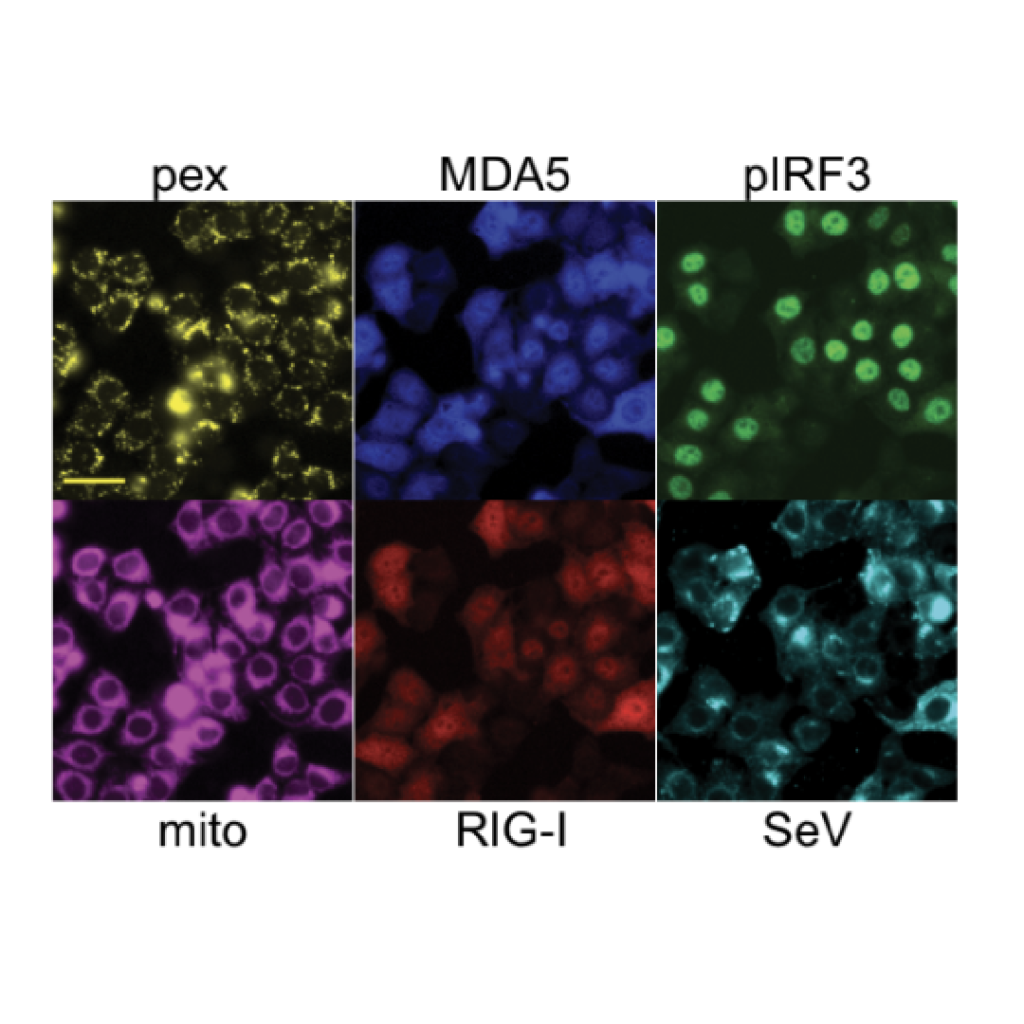
A genome-wide optical pooled screen reveals regulators of cellular antiviral responses
Optical pooled screens pair single-cell phenotypic imaging with large-scale CRISPR or other perturbations, providing rich information about complex processes in huge numbers of cells at a time. Rebecca Carlson, Paul Blainey and our collaborators have expanded the scope of optical pooled screens genome-wide and deployed the technology to explore how cells regulate their responses to RNA virus infection. The genome-wide screens revealed previously unrecognized aspects of viral sensing in cells, and demonstrated how conventional and machine learning-enhanced computer vision-based phenotyping and genome-wide pooled CRISPR screening expand the scope of functional genomics research.
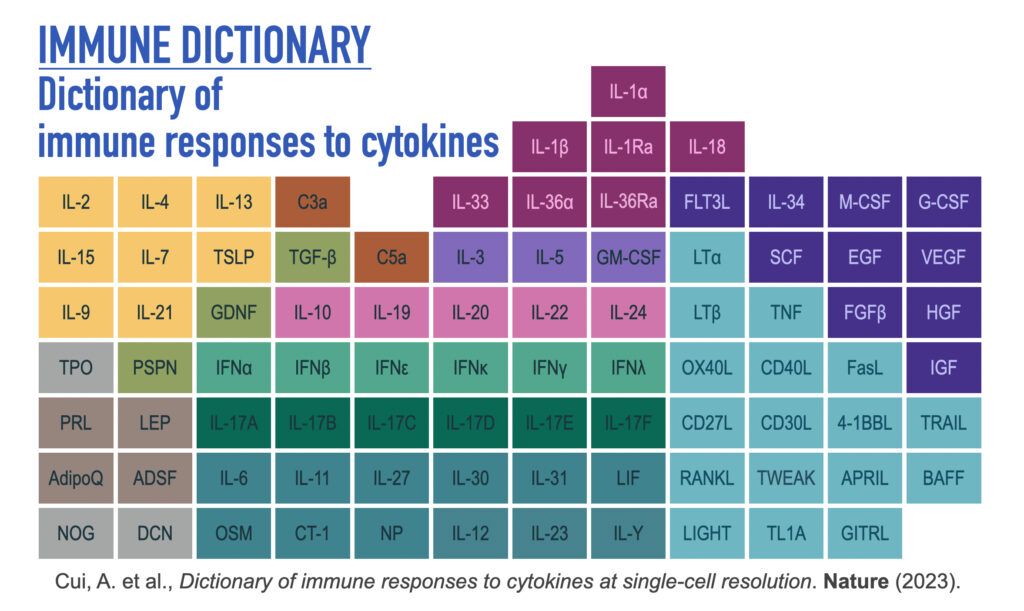
Dictionary of immune responses to cytokines at single-cell resolution
Cytokines mediate cell–cell communication in the immune system and represent important therapeutic targets. A myriad of studies have highlighted their central role in immune function, yet we lack a global view of the cellular responses of each immune cell type to each cytokine. To address this gap, we created the Immune Dictionary, a compendium of single-cell transcriptomic profiles of more than 17 immune cell types in response to each of 86 cytokines (>1,400 cytokine–cell type combinations) in mouse lymph nodes in vivo. A cytokine-centric view of the dictionary revealed that most cytokines induce highly cell-type-specific responses. For example, the inflammatory cytokine interleukin-1β induces distinct gene programmes in almost every cell type. A cell-type-centric view of the dictionary identified more than 66 cytokine-driven cellular polarization states across immune cell types, including previously uncharacterized states such as an interleukin-18-induced polyfunctional natural killer cell state. Based on this dictionary, we developed companion software, Immune Response Enrichment Analysis, for assessing cytokine activities and immune cell polarization from gene expression data, and applied it to reveal cytokine networks in tumours following immune checkpoint blockade therapy. Our dictionary generates new hypotheses for cytokine functions, illuminates pleiotropic effects of cytokines, expands our knowledge of activation states of each immune cell type, and provides a framework to deduce the roles of specific cytokines and cell–cell communication networks in any immune response.